Step-by-step explanation:
it would be
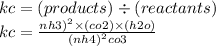
this the expression however when you are working out Kc do not include nh4^2co3 as it is a solid and it's concentration remains constant so remember to remove the nh4^2co3 and usage would just have the top part to work out ok