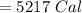Answer:
The correct answer is "5217 Cal".
Step-by-step explanation:
The given values are:
Specific heat,
c = 3.76 cal/g°C
Mass,
m = 25.0 g
Initial temperature,
T₁ = 21.5°C
Final temperature,
T₂ = 77.0°C
Now,
The heat energy will be:
⇒
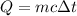
On substituting the given values, we get
⇒
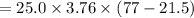
⇒
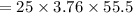
⇒