Answer: W = 6.4x10⁻²¹J
Step-by-step explanation: Work is the energy transferred to or from an object due to the application of a force along a displacement.
The ion Na is gaining energy to move from a lower potential to a higher one.
For this to happen, the work done is calculated as
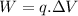
in which
W is work in Joules (J)
q is charge in Coulomb (C)
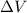 is potential difference in Volts (V)
is potential difference in Volts (V)
The ion Na has one positive charge. In coulombs, the charge is
q = 1.6x10⁻¹⁹C
The potential difference in Volts is
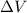 = 40x10⁻³ or 4x10⁻²V
= 40x10⁻³ or 4x10⁻²V
Calculating
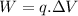
W = 1.6x10⁻¹⁹. 4x10⁻²
W = 6.4 x 10⁻²¹ J
The work done to move an ion of sodium from the exterior to the interior is 6.4 x 10⁻²¹ J.