Answer:
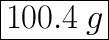
Step-by-step explanation:
In order to find the mass of the gas we must first find the number of moles of the gas at STP ( standard temperature and pressure) and use it together with the molar mass of the gas.
In order to find the number of moles from the volume given we use the formula;
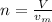
where
V is the volume of the gas
n is the number of moles
v(m) is the molar gas volume at stp which is
22.4 L/mol
From the question
V = 56.2 L
We have;
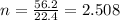
n = 2.51 moles
Now we use the formula m = M × n to find the mass of the gas
where
m is the mass
M is the molar mass
Molar mass of Argon = 40 g/mol
We have
m = 40 × 2.51 =. 100.4
We have the final answer as:
100.4 g