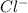Answer: The four ions formed are
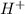 ,
,
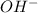 ,
,
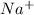 and
and
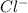
Step-by-step explanation:
Electrolysis is the process where electricity is used to drive chemical reactions.
In the electrolysis of aqueous solution of sodium chloride, hydrogen gas produces at the cathode which is a negative electrode and chlorine gas produces as the anode which is a positive electrode and the sodium hydroxide remains dissolved in the solution.
The reactions will be :
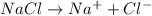
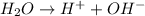
Hence, electrolysis of an aqueous solution of sodium chloride lead to the formation of four ions,
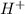 ,
,
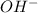 ,
,
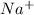 and
and