Answer:
2.5 moles of H2 will be produced by
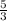 moles of Al
moles of Al
Step-by-step explanation:
The given equation is a balanced equation
2Al +6HCl → 2AlCl3 + 3H2
As per this reaction, 2 moles of Al are used to produce 3 moles of H2
1 mole of H2 will be produced by
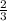 moles of Al
moles of Al
2.5 moles of H2 will be produced by
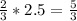 moles of Al
moles of Al