Answer:
Step-by-step explanation:
I'll answer question 3 for you. But just ask one question at a time.
it's really not fair to ask a bunch of questions.
so for 3 we know it's hydroCarbons. or
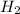 b/c of how Hydrogen is diatomic, C , for Carbon. Next, we know 8 Carbon atoms have attached themselves. so do you happen to know the Lewis diagram for Hydrogen and Carbon? look it up if not. so we know Carbon is 6 on the periodic chart, so it's normally got 6 electrons. I mentioned Lewis structures b/c now you have to picture how the electrons are going to "stick" the atoms together. Recall they like to be in groups of 8 :P so the diatomic Hydrogen is going to stick to one Carbon atom well. Also, Hydrogen is never the central atom, it just "hangs around" :DD , so I added a picture of the Lewis structure for 8 carbon atoms, but, you can see there are double bond connections and the question you asked doesn't say if those are allowed. I feel like the professor has misguided you in this question. b/c HydroCarbons come in a big variety of complex connections, so almost any answer would be correct. but probably 16 is good. but that's not even an option. Maybe they just want you to recognize that Hydrogen is diatomic and comes in twos. so maybe 10 is the best answer. or A)
b/c of how Hydrogen is diatomic, C , for Carbon. Next, we know 8 Carbon atoms have attached themselves. so do you happen to know the Lewis diagram for Hydrogen and Carbon? look it up if not. so we know Carbon is 6 on the periodic chart, so it's normally got 6 electrons. I mentioned Lewis structures b/c now you have to picture how the electrons are going to "stick" the atoms together. Recall they like to be in groups of 8 :P so the diatomic Hydrogen is going to stick to one Carbon atom well. Also, Hydrogen is never the central atom, it just "hangs around" :DD , so I added a picture of the Lewis structure for 8 carbon atoms, but, you can see there are double bond connections and the question you asked doesn't say if those are allowed. I feel like the professor has misguided you in this question. b/c HydroCarbons come in a big variety of complex connections, so almost any answer would be correct. but probably 16 is good. but that's not even an option. Maybe they just want you to recognize that Hydrogen is diatomic and comes in twos. so maybe 10 is the best answer. or A)