Answer:
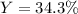
Step-by-step explanation:
Hello there!
In this case, according to the balanced chemical reaction:
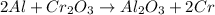
We notice there is a 1:1 mole ratio between Al2O3 and Cr2O3; thus, the following stoichiometric setup is used to compute the theoretical yield first:
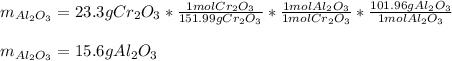
Thus, the percent yield turns out:
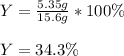
Best regards!