Answer: CONDENSED ELECTRON CONFIGURATION FOR MN2+ =
1.[Ar} 4
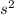 3
3
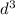
2.This is paramaganetic
Step-by-step explanation:
1. On the periodic table, you first find Mn which is in the "d block section"
The condensed electron configuration of Mn is [Ar} 4
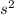 3
3
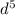 however since the charge is 2+ this means that the atom is losing two-electron. So configuration as stated before is [Ar} 4
however since the charge is 2+ this means that the atom is losing two-electron. So configuration as stated before is [Ar} 4
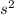 3
3
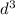
2. This Configuration is paramagnetic since the "d" orbitals are not all filled with electrons.
Hope this helps someone out!