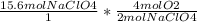Answer:
31.2 moles of O2
Step-by-step explanation:
You have to do stoichiometry to convert moles of NaClO4 to moles of O2.
You have 15.6 moles of NaClO4, you need these to cancel out so you use your coefficient from the equation. Since you are trying to find moles of O2 you put your coefficient of oxygen in the numerator. Multiply across the top & divide by the bottom to get 31.2 moles of O2.