Gay-Lussac's Law
It states that: "At constant volume the pressure of a gas sample is directly proportional to the absolute temperature."
This means that doubling the pressure of the gas will cause its absolute temperature to double.
Mathematically this law is expressed as:
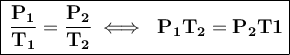
We have that the data is:
P₁ = 0.87 atm
T₁ = 25 °C + 273 = 298 K
T₂ = 50 °C + 273 = 323 K
P₂ = ?
We clear for the final pressure:
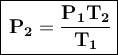
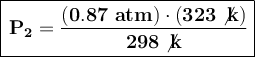

If the temperature increases to 50°C, its new pressure will be 0.94 atm.