Any time we are working with solutions and its molar concentration we will use the equation:
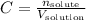
Where C is the concentration, n is the number of moles of the solute and V is the volume of solution.
We can identify such situations when we are working with molarity, molar concentration, solutions with concentration in "M" or "mol/L" units and other cases.
In this case, we can see that we are working with these three quantities:
- We want the volume in mL os a solution, V.
- We have a molar concentration, C, 0.626 M NaOH.
- We have a number of moles of solute we want to get, n, 0.332 mol of NaOH.
So, we have:
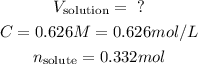
So:
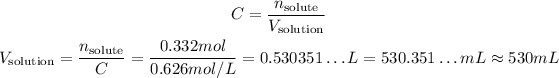
So, the volume we need is approximately 530 mL.