Answer
3.4 L
Step-by-step explanation
Given:
Initial temperature T₁ = 375 K
Final volume of the gas, V₂ = 2.7 L
Final temperature, T₂ = 298 K
What to find:
The volume of the gas in liters at 375 K
Step-by-step solution:
The volume of the gas in liters at 375 K can be calculated using Charle's law formula.
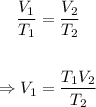
Putting the values of the parameters into the formula, we have;
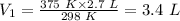
The volume of the gas in liters at 375 K is 3.4 L