1) List known values.
sample: 0.600 mol
Pressure: 1.0 atm
Temperature: 15ºC
Constant: 0.082057 L⋅atm⋅K−1⋅mol−1
List unknown values
Volume:
2) Set the equation and solve for V.
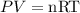
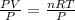
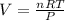
3) Converting to proper units.
15ºC + 273.15 = 288.15 K
4) Plug in known values.
n: 0.600 mol
R: 0.082057 L⋅atm⋅K−1⋅mol−1
T: 288.15 K
P: 1.0 atm
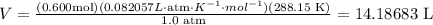
The sample will occupy a volume of 14.19 L.