The change in potential energy ΔU of a system where a charge q moves through a potential difference ΔV is:
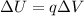
The work needed to move a charge q through a potential difference ΔV is the same as the change in potential energy of the system. Then, replace q=767.1kC and ΔV=567.78V to find the work needed to complete the described process:
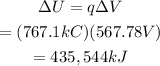
Therefore, the amount of work needed to complete such a process is approximaely 435,544 kiloJoules.