If a body with mass m and specific heat capacity c increases its temperature by ΔT, then the amount of heat Q absorbed by the body in the process is given by:
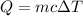
Notice that we know the amount of heat generated by the jogger, as well as its mass and its specific heat capacity, but the increase in temperature is unknown.
Then, isolate ΔT from the equation and replace the values Q=8.2*10^5J, m=73kg and c=3500J/(kgºC) to find the temperature increase of the jogger's body:
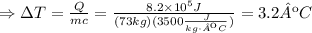
Since the initial body temperature was 37ºC, and the increase in temperature if the heat was not removed is 3.2ºC, then the final body temperature would be:
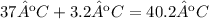
Therefore, if the heat was not removed from the body, its final temperature would be 40.2ºC.