Answer:
0.0104 moles of NO2 will be formed.
Step-by-step explanation:
1st) It is necessary to make sure that the equation is balanced:
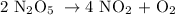
Now we know that 2 moles of N2O5 produces 4 moles of NO2 and 1 mole of O2.
2nd) Using the moles given in the balanced equation, we can calculate the moles of NO2 that will be produced from the completely reaction of N2O5:
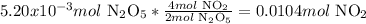
When writing the equation, we can think like this:
From the reaction we know that 2 moles of N2O5 produce 4 moles of NO2 (that is the relation that we have from the reaction) so, when the 5.20x10^-3 moles of N2O5 react completely they will produce a new amount of NO2.
The equation must start with the given value of 5.20x10^-3 moles of N2O5. Then we have to write in the denominator the part of the relation that has N2O5 too (2 moles of N2O5), and then we have to write in the numerator the other part of the relation, in this case, 4 moles of NO2. That is so because the final result will be moles of NO2.
Finally, 0.0104 moles of NO2 will be formed.