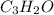ANSWER
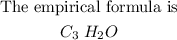
Explanation:
From the question provided, you were told the organic compound contains Carbon, Hydrogen, and Oxygen
Given information
% by composition of carbon = 64.00%
% by composition of hydrogen = 4.03%
Recall that, the organic compound contains carbon, hydrogen, and oxygen, but you were not given the % by the composition of oxygen.
Note that, the % composition of constituents in a compound is 100%. The next thing is to find the % by the composition of oxygen.
Let the % by the composition of oxygen be x
% by composition of carbon + % by composition of hydrogen + % by composition of oxygen = 100%
64.00% + 4.03% + x% = 100%
68.03% + x% = 100%
Isolate x% by subtracting 68.03% from both sides
68.03% + x% - 68.03% = 100% - 68.03%
x% = 31.97%
x = 31.97%
Therefore, % by composition of oxygen in the organic compound = 31.97%
The next thing is to find the empirical formula
Note that, the molar mass of carbon, oxygen, and hydrogen is given in the periodic table
• Molar mass of carbon = ,12 g/mol
,
• Molar mass of oxygen = ,16g/mol
,
• Molar mass of hydrogen = ,1 g/mol
The next thing is to approximate the mole ratio of each element
Carbon = 3
Hydrogen = 2
Oxygen = 1
Therefore, the empirical formula is given below