The number of gold atoms is 1.590x10^23.
- First, with the molar mass of Au2Cl6 (607g/mol) we can find the grams of Au in 80g of Au2Cl6:
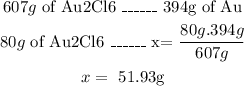
Now we know that there are 51.93g of Au in 80g of Au2Cl6.
- Second, with the molar mass of gold (197g/mol) we can calculate the number of moles in 51.93g of gold:
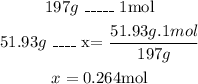
Now we know that there are 0.264 moles of gold in the sample.
- Third, with the Avogadro's number (6.022*10^23) we can calculate the number of gold atoms in the sample:
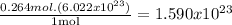
So, the number of gold atoms is 1.590x10^23.