1) Chemical equation.
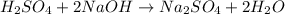
2) Types of reaction.
Synthesis: a compound is made from simpler materials.
Decomposition: a compound is broken down into simpler compounds.
Single replacement: one element that starts out by itself replaces another element in a compound.
Double replacement: positive and negative ions in two compounds switch places.
Combustion: a compound containing Carbon and Hydrogen combines with Oxygen gas and produces carbon dioxide and water.
3) The chemical equation above is a double displacement where H+ and Na+ switch places.
.