Answer: Q = -1335kJ ( exorthemic reaction)
Calculations:
Given the following parameters :
• Mass =75kg = 75*1000 = ,75000g
,
• Temperature final = 32.5 °C
,
• Temperature initial = 37.5°C
,
• Specific heat of human body ,(C,) = 3.56J/g
,
• We will use the formula Q = m*c*∆T to calculate total heat lost.
,
• Take note that this is an ,exorthermic reaction. ,therefore q <0
1. Calculate ∆T
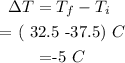
• Therefore ∆T = -5°C
2. Calculate Q
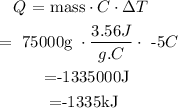
• Therefore ; total heat lost from an average human when the body temperature drops from 37 °C to 32.5= -1335kJ