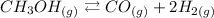
A) Equilibrium constant is usually written as:
![\begin{gathered} aA\rightarrow bB\text{ } \\ k=([B]^b)/([A]^a) \\ \\ K=([CO]*[H2]^2)/([CH3OH]) \end{gathered}](https://img.qammunity.org/qa-images/2023/formulas/chemistry/college/i9clabh0arp91tts3ukw.png)
We will firstly determine the concentrations of the gases:
![\begin{gathered} c=(n)/(V) \\ c:concentration \\ n:moles \\ V:volume \\ \text{ }[CO]=(1.38)/(10.L)=0.138M \\ \\ \text{ }[H2]=(1.26)/(10.0L)=0.126M \end{gathered}](https://img.qammunity.org/qa-images/2023/formulas/chemistry/college/iiw9tu2abjkrdcwkfdu4.png)
B) By substituting what we know in the first equation to find out our unknown we have:
![\begin{gathered} 0.04=\frac{[{0.138][0.126]^2}}{x} \\ x=0.055M \\ \end{gathered}](https://img.qammunity.org/qa-images/2023/formulas/chemistry/college/31hywh9qtiw3ccesn4ux.png)
To determine the number of moles of CH3OH, we multiply the molar concentration by 10L
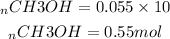
At equilibrium we have 0.55mol of CH3OH
C) If you want the equilibrium to to be shifted to the right in the direction of the product you can remove the CO from the ballon. According to Le Chateliers principle, the foward reaction is favored if the concentration of the the product is deecreased so that more product can be formed.