Answer:
d) 1.2 x 10²⁴ (molecules).
Step-by-step explanation:
The Mole => Avogadro's Number.
Remember that the Avogadro's number is 6.022 x 10²³ molecules/mol. This means that we have 6.022 x 10²³ molecules in 1 mol. We want to find how many molecules there are in 2.0 moles of N2 gas, so the calculation will look like this:
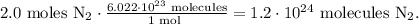
To convert from moles to molecules you just have to multiply the number of moles by Avogadro's number, so in this case, the answer would be d) 1.2 x 10²⁴ (molecules).