It is given that a 5% alchohol solution needs to be prepared.
It is given that 60ml of 60% pure alchohol solution.
So let x ml of pure water be added, so it follows:
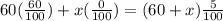
Solve for x to get:
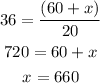
660 ml of pure water needs to be added to get the 5% solution.
b) You will need 660ml of pure water to obtain 720ml of the desired 5% solution.