Answer
C. 4
Step-by-step explanation
Given:
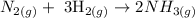
Moles of hydrogen = 12 moles
Required: Moles of nitrogen
Solution
Use the stoichiometry to find the moles of nitrogen
The molar ratio between hydrogen and nitrogen is 3:1
Therefore the moles of N2 = 12 moles x (1/3) = 4 moles