Answer
The pH of the solution is 3 and this will form an acid.
Step-by-step explanation
If the hydrogen ion concentration, [H⁺] of an unknown solution is 7.89E-4, then the pH of the solution can be calculated using the pH formula below.
pH = -log[H⁺]
Putting [H⁺] = 7.89E-4, we have;
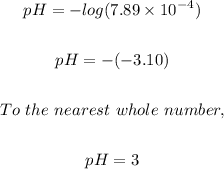
The pH of the solution is 3 and this will form an acid because the pH value falls between the acidic range, (0.1 to 6.99)