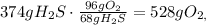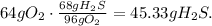First we need to balance the redox-equation:
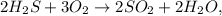
We calculate the number of grams of H2S and O2 using the atomic mass:
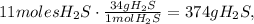
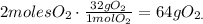
68 g of H2S (2*atomic mass of H2S) produces 96 g of O2 (3*atomic mass of O2):
Then, we calculate the limit reagent and excess reagent, following the calculation: