Answer
2612 mL
Step-by-step explanation
Given:
The initial volume, V₁ = 300 mL
The initial pressure, P₁ = 1.2 bar
The initial temperature, T₁ = 25⁰C = (25⁰C + 273) = 298 K
The final pressure, P₂ = 0.45 bar
The final temperature, T₂ = 700⁰C = (700⁰C + 273) = 973 K
What to find:
The final volume, V₂ of the gas at 0.45 bar pressure and 700⁰C.
Step-by-step solution:
The question is a volume, pressure, and temperature relationship.
The final volume, V₂ of the gas at 0.45 bar pressure and 700⁰C can be calculated using the Combine gas law equation.
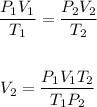
Substituting the values of the parameters into the formula
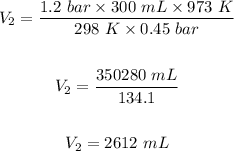
Hence, the volume of the hydrogen gas sample at 0.45 bar pressure and 700⁰C is 2612 mL.