Answer:
The [OH−] is 5.88x10^-7M.
Step-by-step explanation:
1st) It is necessary to calculate the pH of the solution using the pH formula and replacing the concentration of H3O+:
![\begin{gathered} pH=-log\left[H_3O+\right] \\ pH=-log(1.7×10−8) \\ pH=7.77 \end{gathered}](https://img.qammunity.org/qa-images/2023/formulas/chemistry/college/brl7lcsycejaq4kpdiwx.png)
Now we know that the pH is 7.77.
2nd) Now we can calculate the pOH of the solution using the relation between pH and pOH:
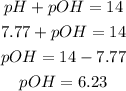
Now we know that the pOH of the solution is 6.23.
3rd) Finally, we can calculate the [OH−] using the pOH formula and replacing the value of pOH:
![\begin{gathered} pOH=-log\left[OH−\right] \\ 6.23=-log\left[OH−\right] \\ 10^((-6.23))=\left[OH−\right] \\ 5.88*10^(-7)=\left[OH−\right] \end{gathered}](https://img.qammunity.org/qa-images/2023/formulas/chemistry/college/9firuhff4uu9wtocqqze.png)
So, the [OH−] is 5.88x10^-7M.