Step 1 - Understanding % concentration in mass
% concentration in mass expresses the concentration of a solute by its percentage, in mass, in relation to the total mass of the solution.
Mathematically, we can find this percentage by the following relation:
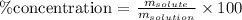
Step 2 - Caculating the % concentration for the given solution
According to the exercise:
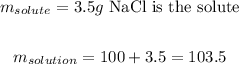
Setting these values in the equation:
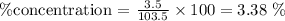
Answer: the % concentration of NaCl in this solution is 3.38%.