We have a reaction between potassium oxide and water. They already present us with the chemical equation but we must balance it.
For this we add the coefficient two in front of the KOH molecule, in this way we will have on each side of the reaction:
K--->2 atoms
O---> 2 atoms
H---> 2 atoms
So, the chemical balanced equation will be:
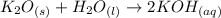
The subscript "s" refers to the solid state, "l" to a liquid state, and "aq" to a solution of the solute in water.