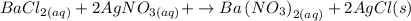2. (a) We are told that it is a double displacement reaction, so there will be two substitutions and Cl will exchange places with NO3.
Now, that will be the full reaction but we need to balance it. Count the atoms of each element on each side of the reaction. In the figure we can see that Chlorine, Nitrogen and Oxygen are not balanced.
We place the coefficient 2 in front of the AgCl molecule to balance the chlorides. You can see the picture.
Now we will balance the nitrogens. There are two nitrogens in the products. We place the coefficient two in the AgNO3 molecule. In the figure I will make the update.
Now the equation is balanced, so the balanced reaction will be: