Firstly, we need to convert the 100 g of AlCl₃ to number of moles, using:
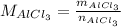
The molar weight of AlCl₃ is calculating consulting the atomic weights:
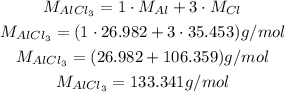
Thus:
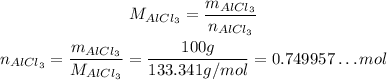
For each 2 moles of AlCl₃, we produce 3 moles of CaCl₂. So:
2 mol AlCl₃ --- 3 mol CaCl₂
0.749957... mol AlCl₃ --- x

Now, we convert back to grams, but now we need the molar weight of CaCl₂:
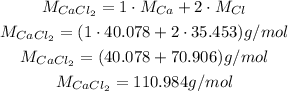
Thus:
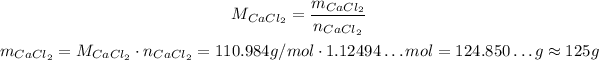
So, 100 g of AlCl₃ will produce approximately 125 g of CaCl₂.