Step 1 - Finding the concentration in g/L
We know the density of the solution is 1.072 g/ml, which means there are 1.072 g in each ml of solution. This mass corresponds to both water and ethyleneglycol.
Since the percentage in mass of ethyleneglycol is 56%, we can calculate how much ethyleneglycol there is in 1 ml of solution:
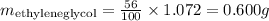
The concentration of ethyleneglycol would be thus 0.6 g/ml.
To convert it to g/L, we just have to multiply it by 1000, because 1L = 1000ml:
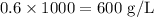
The concentration of ethyleneglycol is this solution is thus 600 g/L.
Step 2 - Converting this concentration to mol/L
To convert now to mol/L (M), we just have to divide the concentration by the molar mass of ethyleneglycol (62.07 g/mol):
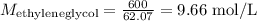
Note the concentration is a rather high one. We will have to dilute it.
Step 3 - Preparing a 0.350 M solution
We know the final volume of the solution must be 500 ml, and we also know its final concentration (0.350 M). Since we already know its inicial concentration as well, we can use the following formula for dilutions:
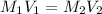
Plugging the values in the equation:
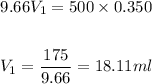
Step 4 - Describing how to prepare the solution
As we have calculated in step 3, we would need 18.11 ml of the original solution (9.66 M) for the dilution. We would then add water untill we get the final volume of 500 ml (add 481.89 ml of water)
The resulting solution would be a 500 ml solution of 0.350 M ethyleneglycol.