A real-world example that can demonstrate the relationship between the variables temperature and volume is, for example, when we inflate a balloon for a party.
- First, we fill the ballon with a gas (air).
- Second, if we tie the balloon and heat it with some source of heat, we will see that the ballon begins to increase in volume.
So, as we increase the temperature of the gas inside the ballon, the balloon increases in volume.
This happens because temperature and volume are variables directly proportional through the Ideal Gas Law, whose formula is:
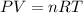
In the formula:
P is the pressure of the gas.
V is the volume of the gas.
n is the number of moles.
R is the constant of the gases.
T is the temperature of the gas.