Chemistry => Stoichiometry => Limiting reactant
The limiting reactant corresponds to the reactant that produces the least amount of moles of products, that is, it will be the reactant that theoretically reacts completely.
To find the limiting reactant we will first calculate the moles of each reactant. For that, we divide the given mass by the respective molar mass of the compounds.
Molar Mass Al = 26.98g/mol
Molar Mass CuCl = 98.999g/mol
Moles Aluminum
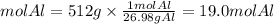
Moles Copper Chloride
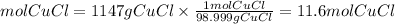
Now, to find the limiting reactant we divide the moles found in each compound between the stoichiometry coefficient from the balanced equation.
So, we will have:
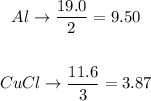
The compound with the smallest quotient will be the limiting reactant.
So, the limiting reactant will be CuCl
Answer: B. CuCl