Answer:
The new volume is 480.75L.
Step-by-step explanation:
The given information from the exercise is:
- Initial amount of moles (n1): 5.47 moles
- Initial volume (V1): 186.5L
- Final amount of moles (n2): 14.1 moles
1st) We have to replace the values of V1 and n1 in the Idea Gases formula:
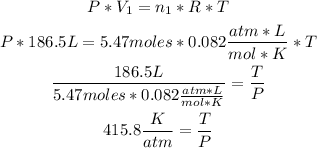
Now we have a value for the relation T/P that remains constant.
2nd) Finally, we have to replace the value of the relation T/P and the amoun of moles n2:

So, the new volume is 480.75L (rounded to 481L).