Answer:
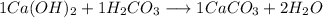
Explanations:
Given the unbalanced acid/base reaction expressed as:
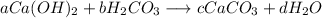
The equation is balanced if the number of moles of element at the reactant is equal to that of the product.
Next is to determine the values of the constants a,b, c, and d by equating the number of moles of the element on both sides.
For the Calcium element:
a = c .................................. 1
For the Oxygen element
2a + 3b = 3c + d ......................... 2
For the Hydrogen element
2a + 2b = 2d
a + b = d .......................... 3
For the carbon element
b = c ....................... 4
Substitute equation 1 and 4 into 3 to have:
c + c = d
2c = d
d = 2c ...................... 5
Substitute equations 1, 4, and 5 into the unbalanced equation to have:
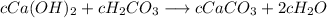
Cancel out the constant "c" from both sides of the equation to have:
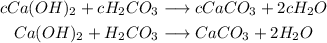
This gives the balanced chemical equation