To answer this question, we have to use calcium oxide molar mass. It is 56.1g/mol, which means that 1 mol of this compound is 56.1 grams. Use this to find how many moles are there in 5.45grams of calcium oxide:
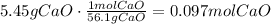
It means that for this mass, there are 0.097 moles of CaO.