ANSWER
b. adding to the internal energy only
Step-by-step explanation
The 1st Law of Thermodynamics states that the total change in the internal energy of a system, ΔU, is equal to the sum of the net heat transfer into the system, Q, and the net work done on the system, W,
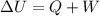
As we can see in this equation, we can increase the internal energy of a system by adding heat to the system or doing work to the system.
So, if heat is added to a system it will increase the internal energy of the system.
Hence, the heat goes into adding to the internal energy only.