We are asked to determine the density of the cork. To do that we will use the following formula:
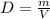
Where:
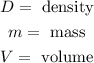
The volume of the cork is the same as the volume that it displaced of water, therefore, we have:
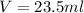
Now, we substitute the values and we get:
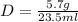
Solving the operations:
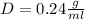
Therefore, the density of the cork is 0.24 g/ml.