Answer:
The number of moles of oxygen atoms that have a mass equal to the mass of a glass of water = 3.1 moles
Explanations:
The mass of oxygen atom = The mass of glass water = 0.050kg
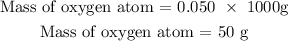
Use the formula below to calculate the number of moles of oxygen atoms
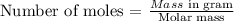
Molar mass of oxygen = 16 g/mol
Mass of oxygen atom = 50g
Substitute these values into the formula above
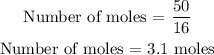
The number of moles of oxygen atoms that have a mass equal to the mass of a glass of water = 3.1 moles