Step 1 - Discover the relation between moles and volume
The gas state equation states that:
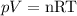
Since the exercise says that both pressure and temperature will remain the same ("under the same conditions"), and R is already a constant, we can obtain a direct relation between moles and volume:
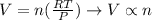
Since R, T and P will not vary, the quocient in parenthesis is a constant. Therefore, the volume will be directly proportional to the number of moles of gas.
Step 2 - using the relation to solve the problem
Since, as we saw, volume and moles are directly proportional to each other, we can set the following proportion to discover the new volume:
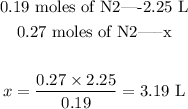
So, the volume of 0.27 moles of N2 gas, under the same conditions, will be 3.19 L.