1) Balance the chemical equation.
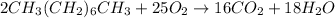
2) Convert grams to moles
2.1-Grams of octane to moles of octane (CH3(CH2)6CH3)
The molar mass of octane is 114.2285 g/mol
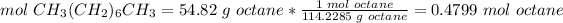
2.2-Grams of O2 to moles of O2
The molar mass of O2 is 31.9988 g/mol.
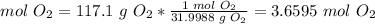
2.3-Grams of CO2 to moles of CO2.
The molar mass of CO2 is 44.0095 g/mol.
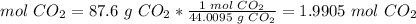
3) Which is the limiting reactant?
3.1-How many moles of octane do we need to use all of the O2?
The molar ratio between octane and O2 is 2 mol octane: 25 mol O2.
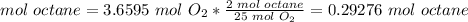
We need 0.29276 mol octane and we have 0.4799 mol octane. We have enough octane. This is the excess reactant
3.2-How many moles of O2 do we need to use all of the octane?
The molar ratio between octane and O2 is 2 mol octane: 25 mol O2.
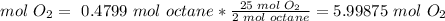
We need 5.99875 mol O2 and we have 3.6595 mol O2. We don't have enough O2. This is the limiting reactant.
4) Moles of CO2 produced from the limiting reactant.
The molar ratio between O2 and CO2 is 25 mol O2: 16 mol CO2.
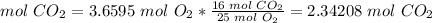
5) Percent yield of CO2
Actual yield: 1.9905 mol CO2
Theoretical yield: 2.34208 mol CO2
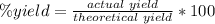
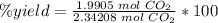
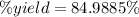
The percent yield of carbon dioxide is 85.0%.
-