To answer this question, we have to use the following formula:
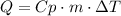
Where Q is the heat, Cp is the specific heat, m is the mass and ΔT is the difference in temperature.
In this case, we know the heat, the mass and the difference in temperature and we need to find the specific heat. Solve the given equation for Cp:
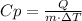
Replace for the given values and solve:
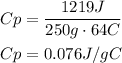
It means that the specific heat of the metal is 0.076J/g°C.