Answer:
0.268grams
Explanations:
According to the Avogadro's constant
1 mole of an atom = 6.02 * 10^23 molecules
Given the following
atoms of calcium = 4.02 x 10^21 atoms
Determine the moles of calcium

Determine the mass of Calcium
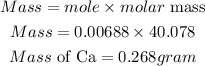
Hence the required grams in 4.02 x 10^21 atoms of calcium is 0.268grams