ANSWER
The theoretical yield of Fe is 109.64 grams
Step-by-step explanation
Given that;
The mass of Fe2O3 is 192 grams
The mass of CO is 82.5 grams
Follow the steps below to find the theoretical yield of Iron
Steps 1; Find the number of moles of Fe2O3 and CO using the below formula
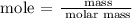
Recall, that the molar mass of Fe2O3 and CO are 159.69 g/mol and 28.01 g/mol

Step 2; Determine the limiting raectant of the reaction
To determine the limiting reactant, divide the number of moles by the co-efficient of each reactant
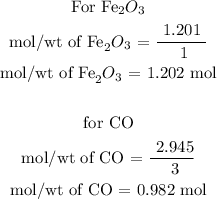
From the calculations, the limiting reactant of the reaction is CO
Step 3; Find the number of moles of Fe using a stoichiometry ratio
Let x represents the number of moles of Fe
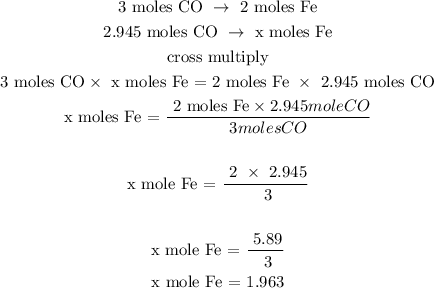
The number of moles of Fe is 1.963 moles
Step 4; Find the theoretical yield of Fe
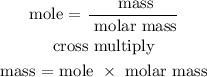
Recall, molar mass of Fe is 55.845 g/mol
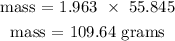
Therefore, the theoretical yield of Fe is 109.64 grams