Since we want the pressure to remain constant we have to use Charles Law of gases, that relates the volume and the temperature at a constant pressure:
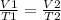
In this case, we know the values of V1, T1 and V2, which are 10L, 25°C and 6.0L. Using these values we have to find T2:
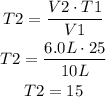
The gas has to be stored at 15°C.