Answer:
7.60grams
Explanations:
The formula for calculating the molarity of a solution is expressed as;
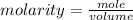
Given the following parameters
molarity of solution = 0.250M
volume of sample = 200mL
Substitute to determine the mole of the solute
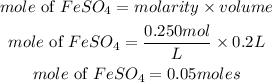
Determine the required mass of FesO4
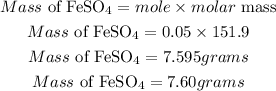
Hence the mass of FeSO4 is 7.60grams