Answer:
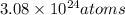
Explanations:
From the question, we are given the following parameters
Moles of Carbon = 5.11 moles
Acording to the Avogadro's constant;
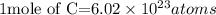
The number of atoms contained in 5.11 moles of Carbon will be expressed as:
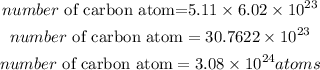
Therefore the number of carbon atoms that are in 5.11 moles of C is
3.08 * 10²⁴atoms